[Main ingredients]
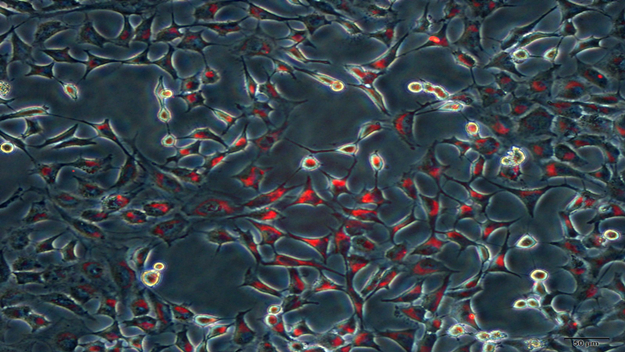
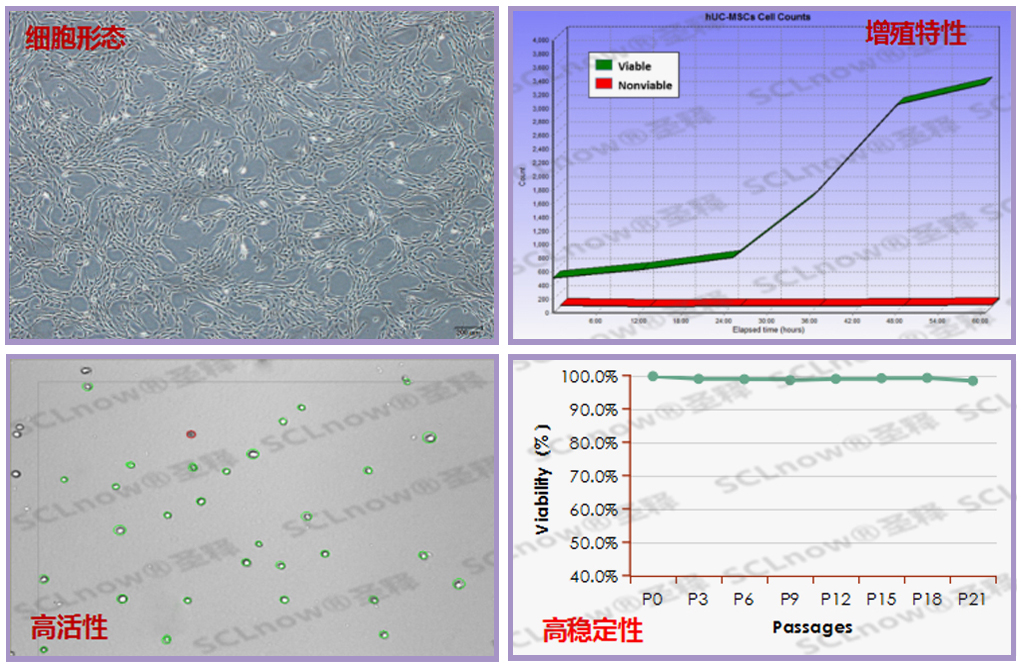
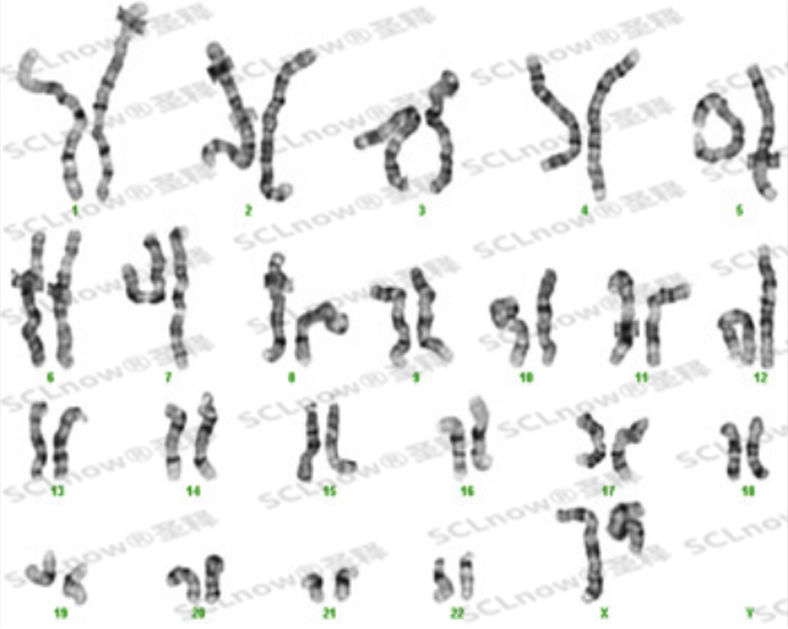
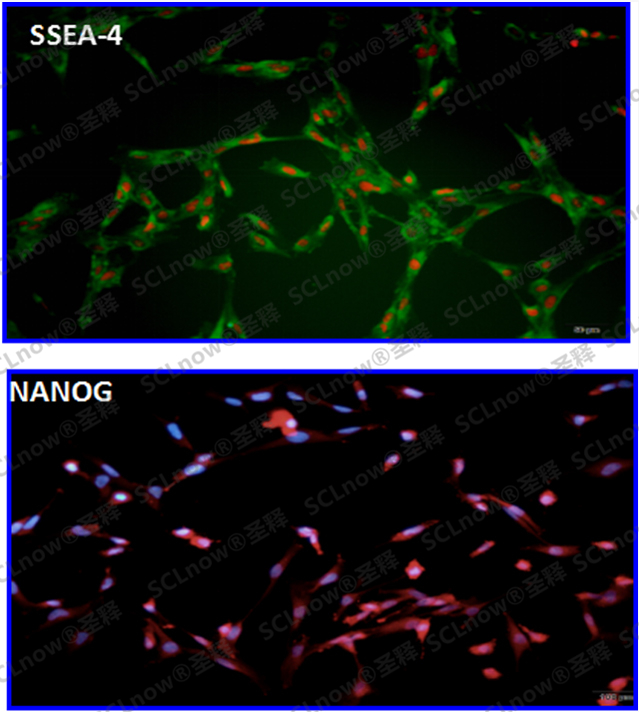
UC-MSC secretory proteins, cytokine etc.
Accessories: water for injection
[Characteristics]
At roomtemperature, this product is in form of liquid in color from light pink tolilac without fragrance
[Specifications]
8ml:40mg。
[Usage and dosage]
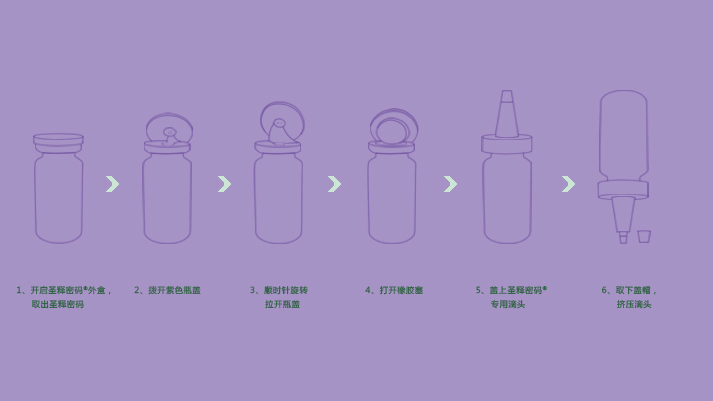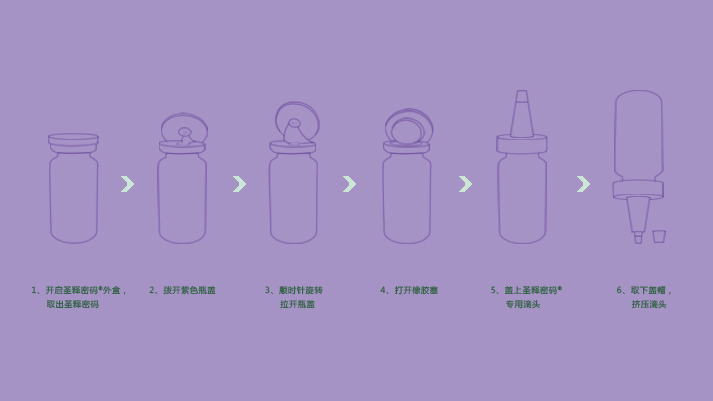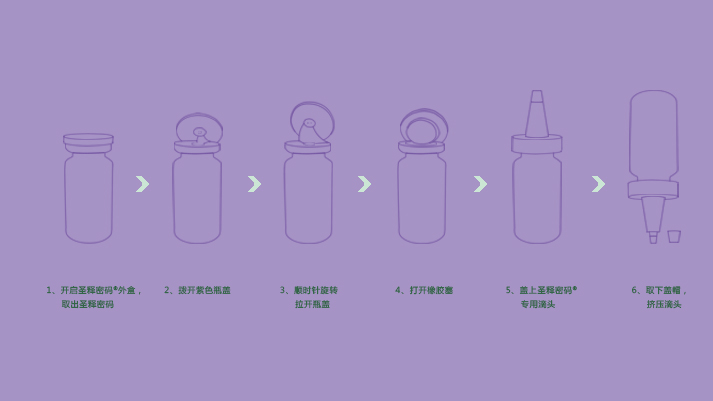
Afterthe skin is cleaned, apply a proper amount of this product evenly to therequired skin.
Because this product has UC-HA,UC-collagen,UC-MSC secretory proteins, cytokine etc., please keep the remainder in refrigeratorafter use and preserve in freezer at -20 ℃ for along-term storage
[Efficacy anddynamics]
1.Nanoscale components are easy for skin toabsorb. UC-HA promotes the proliferation and differentiation of epidermal cells,reduces fine lines, keeps the dermis in 25%-30% moisture, scavenges oxygen freeradicals, reconstructs, repairs and regenerates the skin.
2.Nanoscalecomponents are easy for skin to absorb. UC-collagen has a natural moisturizingfunction. The three spiral structure reaches the stratum corneum and locks themoisture. It nourishes the skin, strengthens the skin metabolism, maintains theintegrity of the skin fiber and makes the skin achieve the function of skinlightening, tightening and moisturizing.
3.Nanoscale components are easy for skin toabsorb, and on the skin surface, form an active polymer screen membranecomposed of UC-MSC secretory proteins and cytokine to activate skin cells inbasal skin layer from the dormancy state to the best active state of growthfactors.
4.UC-MSC cytokinescan deeply reconstruct from the dermis to cuticles, which stimulate fiber cellsto form collagen and elastin. The reconstruction promotes the skin metabolismand recharges the damaged skin cells with necessary energy and nutrition. Itstrengthens the regular skin functions including anti-oxidation,anti-ultraviolet, anti-bacteria and restores the normal metabolism forall-around skin repair and maintenance
5.Small molecule functional proteins and cytokines canmake cells restored to the young state, improving transport function andexchange ability of the cell membrane materials. They improve the respiratoryfunction inside a cell and promote the active transport of cell nutrients fromoutside into the cell in order to increase the nutrition inside the cell. Thispromotes dermal cells to secrete and synthesize functional molecules such ascollagen, polysaccharides, glycoproteins etc. These functional moleculesenhance cell metabolism and make the subcutaneous dermis tissue plump and themuscle fibers evenly and tightly laid out to prolong cell senescence, and hencereduce and eliminate wrinkles.
[Adapt to skin]
1. All types of Oily, mixed, dull, water deficient andlarge pores skin
2. Treatment on all kinds of acne, pimple skinand hormone dependent dermatitis
3. Dull, rough and lusterless skin caused by workingpressure or lack of sleep etc.
4. Skin laxity, small wrinklesand lack of elasticity caused by degenerative aging
[Caution]
1. People with severeallergic constitution should use cautiously
2.People should use cautiously in case of red, swollen, hotitching, painful skin mucous
membrane and eczema caused by bacteria or virus.
[Warning]
1. Thisproduct contains bio-active components and should be transported with coldchain at -20℃ and used within the period of validity.
2. In caseof adverse reaction after use, it should be treated in time.
3. Thisproduct cannot replace pharmaceuticals
[Storageconditions]
Sealed preservation at 2-8 ℃ or -20 ℃
[Term ofvalidity]
Theduration of preservation is 30 days at 2-8℃ or six months at-20℃.
[Standard]
Conform to quality control system of GB/T19001-2016/ISO9001:2015version
[Approval Number]
016ZB17Q30654R1M;ABZB17Q30194R1M
[Manufacturer]
CompanyName:SCLGL® Regernative Medicine Space
Address: SCLnow Fairland,Tianan Golf,NO.39,Dongwei road,Chaoyang District,Beijing,
Postcode: 100018
Telephone: 010-010-5511