SCLGL® Regenerative Medicine Space entrusts a third-party international testing agency to carry out safety testing and stem cell quality standards review for iSCLstem cell® and iSCLife® . Testing items include: a variety of viruses, fungi, mycoplasma, chlamydia, endotoxin and other items. Safety test runs through the whole process of specimen collection, cell culture, cryopreservation and recovery of iSCLstem cell® and iSCLife® products.
SCLGL®ID (Birth Paper) of iSCLstem cell® and iSCLife®
-
 Safety of iSCLstem cell® and iSCLife®
Safety of iSCLstem cell® and iSCLife® -
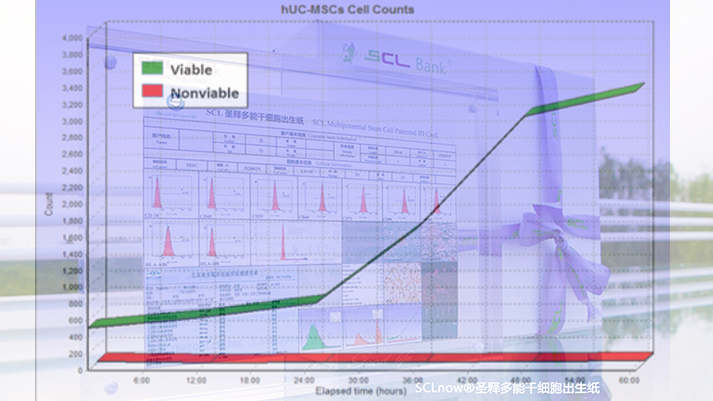 Cell level testing of iSCLstem cell® and iSCLife®
Cell level testing of iSCLstem cell® and iSCLife®In order to guarantee the greatest value of life energy, tests on growth morphology, diameter distribution, activity, roundness, integrity, proliferation characteristics and cell cycle are conducted to ensure cell viability and activity.
-
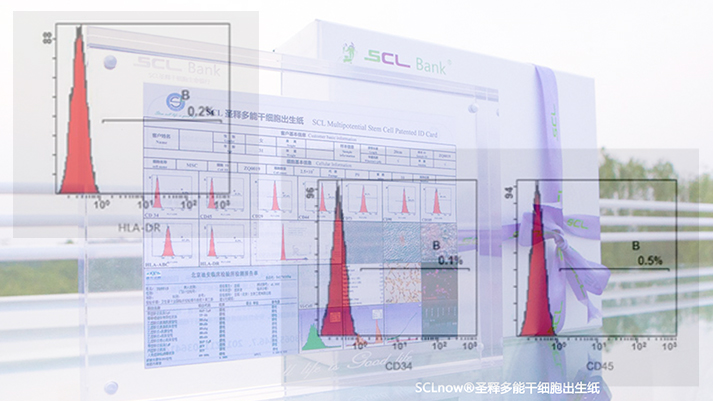 Molecular level testing of iSCLstem cell® and iSCLife®
Molecular level testing of iSCLstem cell® and iSCLife®HLA-DR negative expression indicates iSCLstem cell® and iSCLife® have no need for matching, which is also an important feature in distinguishing iSCLstem cell® and iSCLife® from other types stem cells.
CD34 negative expression indicates that iSCLstem cell® and iSCLife® are Not hematopoietic stem cells.
CD45 negative expression shows iSCLstem cell® and iSCLife® are non lymphocyte and hematopoietic stem cells.
-
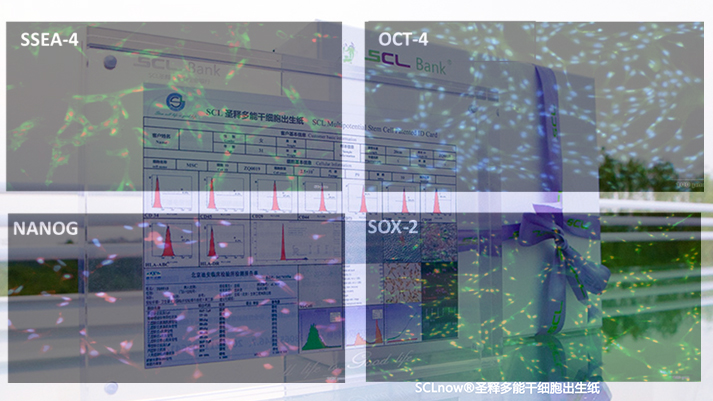 Protein level testing of iSCLstem cell® and iSCLife®
Protein level testing of iSCLstem cell® and iSCLife®The polymorphism protein of OCT-4, SOX-2, NANOG and SSEA-4 of iSCLstem cell® and iSCLife® were detected by immunofluorescence technique and proved to be stable and pluripotent.
-
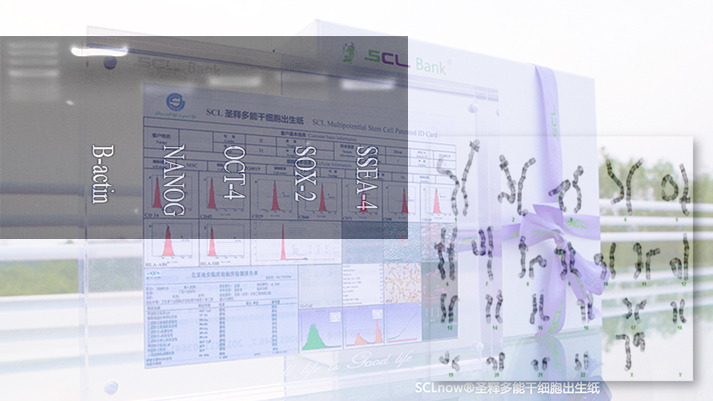 Gene level testing of iSCLstem cell® and iSCLife®
Gene level testing of iSCLstem cell® and iSCLife®Polypotent genes NANOG, OCT-4, SOX-2, SSEA-4 have clear and bright gene bands, which prove the pluripotency of stem cells at the genetic level.
Karyotype analysis of different generations of stem cells demonstrated the stability of chromosomal structure of iSCLstem cell® and iSCLife®. -
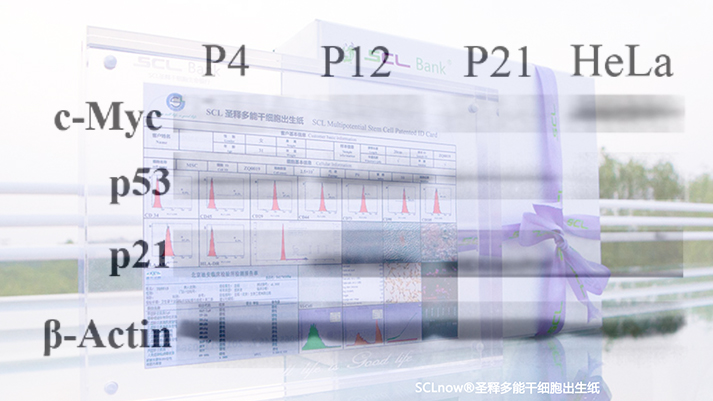 Non tumorigenicity of iSCLstem cell® and iSCLife®
Non tumorigenicity of iSCLstem cell® and iSCLife®iSCLstem cell® and iSCLife® are safe and reliable. There is no tumorigenicity. Umbilical cord derived pluripotent stem cells express tumor suppressor genes in different generations, and do not express proto oncogenes. In addition, umbilical cord pluripotent stem cells did not form tumor colonies and were not tumorigenic in vitro soft agar cloning experiments.
-
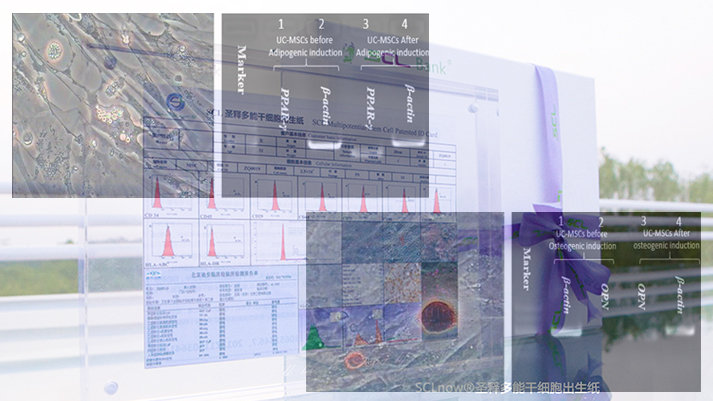 Pluripotency of iSCLstem cell® and iSCLife®
Pluripotency of iSCLstem cell® and iSCLife®Specific staining and differential gene expression showed that iSCLstem cell® and iSCLife® are proven to have the potential of multidirectional differentiation after induction of adipogenesis and osteogenic differentiation in vitro.
All testing items of iSCLstem cell® and iSCLife® are based on years of theoretical and clinical research by scientists of SCLGL® Regenerative Medicine Space and are in accordance with the International Quality Management System Standards of GB/T19001-2008/ISO 9001:2008 SCLGL. Under the premise of ensuring the safety of application, These test results are concluded in terms of the quality, quantity, morphology, purity, activity, stability and pluripotency of umbilical cord pluripotent stem cells detected from the four levels of cell, molecule, protein and gene.
